Moles
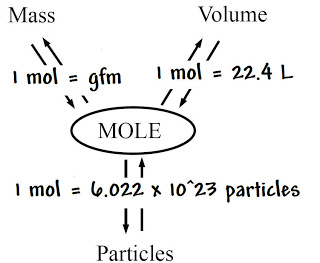
Chemistry can be defined as the identification of the substances found in matter of the universe. Matter is the "stuff" of the world, what the universe is made up of. An item can only be considered matter if it has mass and volume. All matter is made of elements, which are the simplest forms of matter. Some elements stand alone, such as O2 being breathable Oxygen. Other elements are in part of mixtures named compounds, such as NaCl (Sodium Chloride-table salt). A single molecule of NaCl consists of one atom of Na (Sodium) and one atom of Cl (Chlorine), but mix together to form NaCl. However, that is unrealistic because it is very difficult to have a single atom of Na and a single atom of Cl. Therefore, when we refer to "how much" of a substance we have, we use a different unit of measurement named moles. The word mole can be compared to "dozen." The way "a dozen" corresponds to 12, one mole corresponds to multiple things: 6.02x1023 particles, 22.4L of gas at Standard Temperature and Pressure (STP), and mass. 6.02x1023 particles means that for every mole of a substance, there are 6.02x1023 molecules/atoms (a lot). 22.4L means that for every one mole of a gas (at STP), there are 22.4 liters of that gas. 2 moles would be 44.8L, 4 would be 89.6L, and so on. The mass is the only item that isn't constant when referring to moles. One mole of Na has a mass of about 23 grams, while a mole of Pb (Lead) has a mass of 207 grams. This is called mole mass, for obvious reasons.
The Basics
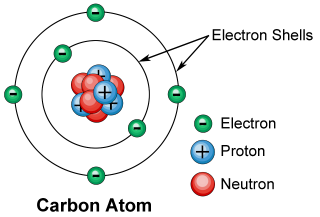
The Period Table is a chart which organizes the 118 known elements of the universe. Generally, it provides information and displays trends on the atomic number, atomic mass, electron configuration, oxidation states, and the chemical symbol, for each of the 118 elements. The main organizing factor of the table is the atomic number. When reading the table left to right, the atomic number is increasing. The atomic number of an element tells you the number of protons in one atom of the element. The electrical charge of one proton is +1, while the electrical charge of an electron is -1. Also, all atoms are neutral, meaning they always have a total charge of zero. Therefore, by knowing the number of protons, we also know the number of electrons, because they must always equal each other in an atom. However, while there are protons and electrons, there are also neutrons. Neutrons have no charge, and are located in the same place as the protons, the nucleus. The number of neutrons in any given element varies. For example, you could have two different types of oxygen, which are different because they have different numbers of neutrons, but because they have the same number of protons (8), they're still both considered oxygen. These are called isotopes. These are variations of the same element, but with different numbers of neutrons. This explains the concept of atomic mass as well. Atomic mass is defined as the weighted average mass of all naturally occurring isotopes. For example, let's say there are only three isotopes of Oxygen, Oxygen-a, and Oxygen-b (that's not how isotopes are named but that will be explained later). Let's say that you find that the natural abundancy of Oxygen-a to be 99.8%, while the natural abundancy of Oxygen-b to be 0.2%. This means that if you were to take a sample of oxygen, you'd find that 99.8% of the sample was Oxygen-a, while 0.2% of the sample is comprised of Oxygen-b. Therefore, to calculate the average mass of Oxygen, you would do a weighted average, and do Oxygen-a's mass times 99.8%, and add it to Oxygen-b's mass times 0.2%. This calculation would give you the atomic mass of Oxygen. Besides atomic mass, we also have mass number. Mass number is simply the number of neutrons plus the number of protons in any isotope of any element. Mass number is also used in naming isotopes, which is also why you usually wouldn't find mass number on the Periodic Table, because there would have to be many, many different isotopes. For example, let's say there are two isotopes of Fluorine that you have. Both obviously have the same number of protons (9), but Fluorine-x has 10 neutrons and Fluorine-y has 12 neutrons. Fluorine-x's mass number would be 19, while Fluorine-y's mass number would be 21. Therefore, Fluorine-x would be named Fluorine-19, and Fluorine-y would be named Fluorine-21. The neutrons and protons are located in the nucleus of the atom, while the electrons are located in the outside. Electrons are actually constantly spinning and turning, so unlike protons and neutrons, there isn't an exact location. Instead there is a zone just outside the nucleus named the electron cloud, which shows all the predicted locations of the electrons. However, that's not all. The electron cloud is organized in separate paths, named shells.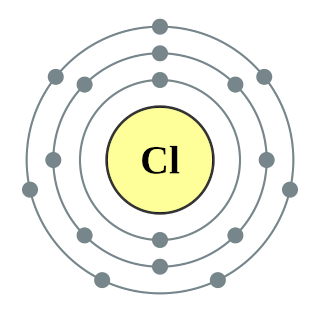 This is associated with the electron configuration of elements. The first shell is located right outside the nucleus, but as the number of electrons increases, more shells get filled, and electrons end up farther and farther away from the nucleus. Each shell holds a different number of electrons, and the expression to find the number of electrons that fit in each shell is 2n2, where n is the shell. For example, the 5th shell holds 2(5)2 electrons, or 50. Let's say you have an atom of Bromine (35 electrons). Its electron configuration would be 2-8-18-7. However, there is also a situation where the number of electrons an element has changes. Almost all elements on the Periodic Table are considered unstable, because most have an incomplete outermost shell. To become stable, these elements need to either gain or lose electrons, to mimic the electron configuration of noble gases, which are considered stable. For example, think of an atom of Fluorine. It has an electron configuration of 2-7, meaning it is only one electron away from becoming stable and "whole," mimicking Neon's electron configuration. When Fluorine eventually gains that electron in some way, it is no longer an atom, but an ion. It becomes an ion because it is no longer neutral, and has a total charge of -1. This is what oxidation states are. Oxygen, for example, has the oxidation state -2. This means that when it becomes an ion, it'll gain a charge of -2, giving it a total charge of -2, because 0+(-2)=-2. Some elements have multiple oxidation states because there's multiple ways they can become whole. For example, Carbon has the oxidation states -4,+4, and +2. This simply means that Carbon would adapt to the circumstances, and would either gain 4 electrons for a total charge of -4, lose 4 electrons for a total charge of +4, or lose 2 electrons for a total charge of +2 (and it would then lose another 2 to become stable).
This is associated with the electron configuration of elements. The first shell is located right outside the nucleus, but as the number of electrons increases, more shells get filled, and electrons end up farther and farther away from the nucleus. Each shell holds a different number of electrons, and the expression to find the number of electrons that fit in each shell is 2n2, where n is the shell. For example, the 5th shell holds 2(5)2 electrons, or 50. Let's say you have an atom of Bromine (35 electrons). Its electron configuration would be 2-8-18-7. However, there is also a situation where the number of electrons an element has changes. Almost all elements on the Periodic Table are considered unstable, because most have an incomplete outermost shell. To become stable, these elements need to either gain or lose electrons, to mimic the electron configuration of noble gases, which are considered stable. For example, think of an atom of Fluorine. It has an electron configuration of 2-7, meaning it is only one electron away from becoming stable and "whole," mimicking Neon's electron configuration. When Fluorine eventually gains that electron in some way, it is no longer an atom, but an ion. It becomes an ion because it is no longer neutral, and has a total charge of -1. This is what oxidation states are. Oxygen, for example, has the oxidation state -2. This means that when it becomes an ion, it'll gain a charge of -2, giving it a total charge of -2, because 0+(-2)=-2. Some elements have multiple oxidation states because there's multiple ways they can become whole. For example, Carbon has the oxidation states -4,+4, and +2. This simply means that Carbon would adapt to the circumstances, and would either gain 4 electrons for a total charge of -4, lose 4 electrons for a total charge of +4, or lose 2 electrons for a total charge of +2 (and it would then lose another 2 to become stable).
