Bonding and Molecules
Bonding, by definition, is when multiple elements come together to make a compound and exchange/share electrons. There are 3 types of bonds: Ionic, covalent, and metallic. First, however, we need to understand how the Periodic Table is divided. At the right portion of the table, there is a "staircase" dividing the metals and nonmetals of the table, as shown below.
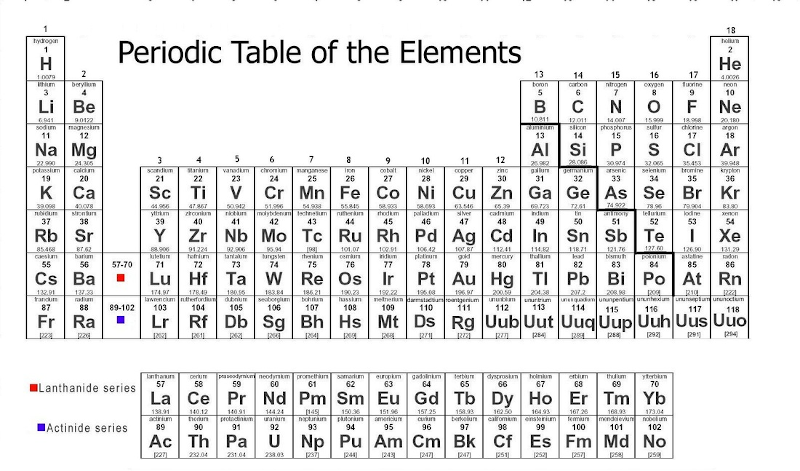
All the metals are directly left of the staircase. Elements classified as "metalloids" are found sitting on the staircase (B, Si, As, Te), as well as Ge and Sb. The first type of bond, ionic, is comprised of metals and nonmentals, and electrons are transferred from the metal to the nonmetal. Ionic compounds are usually hard, good conductors of heat and electricity as liquids, gases, or aqueous solutions, have a high melting point and boiling point, and dissolve in polar substances (like water). The high M.P., B.P., and hardness of ionic compounds is due to the strong attraction between the metal and nonmetal of the bond. Each element has a characterictic named electronegativity, which is simply defined as the strength of attraction that element has for its shared electrons in a bond. This concept of "shared electrons" doesn't have to do with ionic bonding, but it does help explain it better.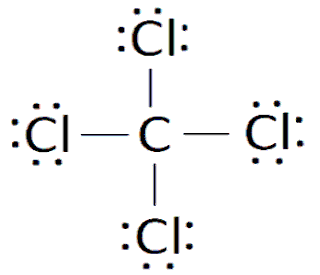 The strong attraction between the metal and nonmetal in an ionic compound is due to the great difference in electronegativity. The higher the difference, the weaker the compound becomes. Ionic compounds are always stronger because nonmetals tend to have high electronegativities, while metals are the opposite, creating a large difference. This is unlike the second type of bond, the covalent bond. A covalent bond is between two nonmetals, and is weak due to the fact that both nonmetals in the compound have high electronegativities, creating a low difference. Also, in an ionic bond the electrons are completely transferred over from the metal to the nonmetal, but in covalent compounds the electrons are shared indefinitely between the two nonmetals. This is because both components of the covalent bond (both nonmentals) need electrons to become stable, so they don't just give them up. This is where the characteristic of electronegativity comes into play, because the nonmetal with the higher electronegativity will have a stronger pull on the shared electrons, and keep them for a longer amount of time. Covalent compounds are soft, poor conductors of heat and electricity, and have low melting points and boiling points. There are multiple types of Covalent bonds, but they all have the same properties. A nonpolar covalent bond is where one nonmetal is bonding with itself, therefore making the electronegativity difference 0. A polar covalent bond is all other scenarios, where the electronegativity difference is greater than 0 but still less than 1.7. The third and final type of bond is metallic bonds, between two metals. Here, electrons are neither transferred nor shared, but just dumped. All metals of the compound lose enough electrons to become stable, creating this large mass of electrons a "sea of mobile electrons."
The strong attraction between the metal and nonmetal in an ionic compound is due to the great difference in electronegativity. The higher the difference, the weaker the compound becomes. Ionic compounds are always stronger because nonmetals tend to have high electronegativities, while metals are the opposite, creating a large difference. This is unlike the second type of bond, the covalent bond. A covalent bond is between two nonmetals, and is weak due to the fact that both nonmetals in the compound have high electronegativities, creating a low difference. Also, in an ionic bond the electrons are completely transferred over from the metal to the nonmetal, but in covalent compounds the electrons are shared indefinitely between the two nonmetals. This is because both components of the covalent bond (both nonmentals) need electrons to become stable, so they don't just give them up. This is where the characteristic of electronegativity comes into play, because the nonmetal with the higher electronegativity will have a stronger pull on the shared electrons, and keep them for a longer amount of time. Covalent compounds are soft, poor conductors of heat and electricity, and have low melting points and boiling points. There are multiple types of Covalent bonds, but they all have the same properties. A nonpolar covalent bond is where one nonmetal is bonding with itself, therefore making the electronegativity difference 0. A polar covalent bond is all other scenarios, where the electronegativity difference is greater than 0 but still less than 1.7. The third and final type of bond is metallic bonds, between two metals. Here, electrons are neither transferred nor shared, but just dumped. All metals of the compound lose enough electrons to become stable, creating this large mass of electrons a "sea of mobile electrons." This "sea" allows the compound to conduct heat and electricity well, as well as be flattened into thin sheets (malleable) and strung into wire (ductile). These three types of bonds are intramolecular forces, meaning the occur within the molecule and hold the parts of the molecule together, but technically, ionic compounds and metallic compounds aren't considered molecules. Covalent compounds are considered molecules, and they have specific shapes as well which determine the overall polarity of the molecule. Some distinct shapes are: Linear, Trigonal Planar, Tetrahedral, Pyramidal, and Angular/Bent. Linear molecules occur when there are no more than 2 atoms of each element. Linear molecules can be either polar or nonpolar, depending on the distrbution of charge throughout the molecule. Trigonal Planar are 2-dimensional and nonpolar and are formed when there is 3 atoms of one element and one of another, such as in BF3. A tetrahedral shape is 3-dimensional and nonpolar, and occurs when there are four atoms of one element, such as in CH4. Pyramidal and Angular/Bent are both abnormalities where the shapes are supposed to be trigonal planar and linear, respectively, but and extra pair of electrons is pushing down on the other elements, bending them and making them polar.
This "sea" allows the compound to conduct heat and electricity well, as well as be flattened into thin sheets (malleable) and strung into wire (ductile). These three types of bonds are intramolecular forces, meaning the occur within the molecule and hold the parts of the molecule together, but technically, ionic compounds and metallic compounds aren't considered molecules. Covalent compounds are considered molecules, and they have specific shapes as well which determine the overall polarity of the molecule. Some distinct shapes are: Linear, Trigonal Planar, Tetrahedral, Pyramidal, and Angular/Bent. Linear molecules occur when there are no more than 2 atoms of each element. Linear molecules can be either polar or nonpolar, depending on the distrbution of charge throughout the molecule. Trigonal Planar are 2-dimensional and nonpolar and are formed when there is 3 atoms of one element and one of another, such as in BF3. A tetrahedral shape is 3-dimensional and nonpolar, and occurs when there are four atoms of one element, such as in CH4. Pyramidal and Angular/Bent are both abnormalities where the shapes are supposed to be trigonal planar and linear, respectively, but and extra pair of electrons is pushing down on the other elements, bending them and making them polar.
